 Official Title Official Title
Secondary Prevention With HMG-CoA Reductase Inhibitor Against Stroke |
 Purpose Purpose
Although hyperlipidemia is not always listed as a risk factor of strokes, inhibition of 3hydroxy-3-methylglutaryl-coenzyme A(HMG-CoA) reductase can decrease the incidence of stroke in the patient with ischemic heart disease.
The neuroprotective mechanism beyond cholesterol lowering should be expected to attenuate
inflammation and atherosclerosis. The present study hypothesizes if pravastatin prevents recurrent stroke
in the ischemic stroke patients with safety.
|
 Primary Outcome Measures: cerebrovascular events Primary Outcome Measures: cerebrovascular events |
 Secondary Outcome Measures: Secondary Outcome Measures:
subtype of ischemic stroke according to the TOAST classification or hemorrhagic stroke, cardiovascular events including myocardial infarction,
all the cerebrovascular and cardiovascular events, death of stroke,
death of cerebrovascular and cardiovascular
Estimated Enrollment: |
3000 |
Study Start Date: |
March 2004 |
Estimated Study Completion Date: |
February 2014 |
|
 Eligibility Eligibility
・Ages Eligible for Study: 45 Years to 80 Years
・Genders Eligible for Study: Both
・Accepts Healthy Volunteers: No |
 Criteria Criteria
― Inclusion Criteria:
・Ischemic stroke except for cardiogenic embolism, from 1 month to 3 years after onset
・Hyperlipidemia and total cholesterol level of 180-240mg/dl without the prescription of
statin within previous 30 days
・ Able to visit outpatient department
・Informed consent on the form.
― Exclusion Criteria:
・Ischemic stroke of other determined cause according to the TOAST classification
・Ischemic heart disease and necessary to use statin
・Hemorrhagic disorders
・Platelet count <=100,000/ul within 3 months prior to study start
・Alanine aminotransferase (ALT) or aspartate aminotransferase (AST)>= 100IU/L
within 3 months prior to study start
・Serum creatinine >=2.0mg/dl within 3 months prior to study start
・A scheduled operation
・The presence of malignant disorder |
 Study Design: Study Design:
・Allocation: Randomized
・Control: Active Control
・
Endpoint Classification: Safety/Efficacy Study
・Intervention Model: Parallel Assignment
・Masking: Open Label
・Primary Purpose: Prevention |
 Intervention Intervention
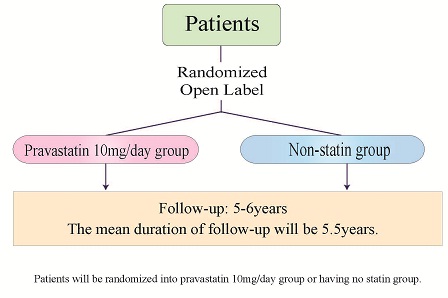 |
 The Status: As of August 2012 The Status: As of August 2012
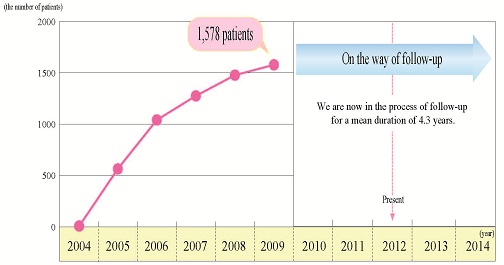 |
 Additional Information: Additional Information:
Clinical Trials.gov
http://clinicaltrials.gov/ct2/show/study/NCT00221104?term=J-STARS&rank=3
Stroke Trial Registry
http://www.strokecenter.org/trials/TrialDetail.aspx?tid=689 |